How does BerriQi repair lung tissue: pro-inflammatory to anti-inflammatory class switching in macrophages.
Lung fibrosis is the thickening and scarring of lung tissue that occurs as a result of lung damage. This damage can have many different causes, including exposure to airborne pollutants, chemotherapy, radiation, or inflammation driven by respiratory pathogens such as COVID-19. When the lungs start to heal, they generate scar tissue in a similar manner to skin when it heals from a cut. Over time, this scar tissue decreases lung elasticity, resulting in stiff lungs and difficulty breathing.
At the cellular level, a major contributing mechanism for lung fibrosis is collagen fibre deposition. When tissue damage is detected in the lung, immune cells respond by depositing collagen fibres around the damaged site. This process is known as tissue remodelling, and over time it thickens the lung tissue, making it inflexible and stiff.
BerriQi, Macrophages and Lung Fibrosis.
BerriQi®, a patented formulation of New Zealand Boysenberries and apple, has been shown to reduce lung fibrosis in preclinical animal models. One of the ways BerriQi does this is by changing the function of macrophages in the lungs, activating these cells in a specific way to help them clean up the excess collagen.
Macrophages are a type of white blood cell that help to defend the body against pathogens. Though best known for their role as the first line of defense against invading pathogens, macrophages also perform a wide variety of roles in development, homeostasis, innate and acquired immunity, and inflammation. Macrophages demonstrate an incredible degree of plasticity and are able to change their function based on the needs of the body. Activated macrophages can be broadly divided up into two classes, M1 and M2, which have unique characteristics and perform different roles in managing pathogens and tissue damage. Macrophages can move between these two classes in response to different stimuli, using a process known as class switching.
M1 macrophages (also known as “pro-inflammatory” or “classically activated” macrophages) have a central role in immunity and actively defend the body against invaders. The main role of these cells is to kill bacterial pathogens or other harmful cells, by engulfing them (phagocytosis) or by releasing cytotoxic (cell-killing) enzymes and chemicals.
These processes are very effective and can rapidly kill invading bacteria before they can establish an infection. However, the release of cytotoxic agents also leads to collateral tissue damage, which the body will need to repair. M1 macrophages undergo class switching after an infection has been cleared, to re-specialise into M2 macrophages that will help repair the damage.
The major role of M2 macrophages (also known as “anti-inflammatory” or “alternatively activated” macrophages) is to repair tissue that has been damaged due to infection or other causes. Unlike M1 macrophages, M2 cells produce healing factors that encourage the regeneration of damaged tissue. They also produce anti-inflammatory signals that help to resolve inflammation and prevent additional damage.
In the context of lung fibrosis, one of the most important functions of M2 macrophages is the production of tissue-remodelling factors that can break down excessive collagen. M2 macrophages produce enzymes called matrix metalloproteinases (MMPs), which can degrade collagen and cause an improvement in the symptoms of lung fibrosis.
Macrophages can switch between these two activation states in response to changing inflammatory conditions and other stimuli. BerriQi can increase the proportion of M2 macrophages present in the lungs, leading to an increase in MMP-driven tissue remodelling to address the underlying cause of lung fibrosis.
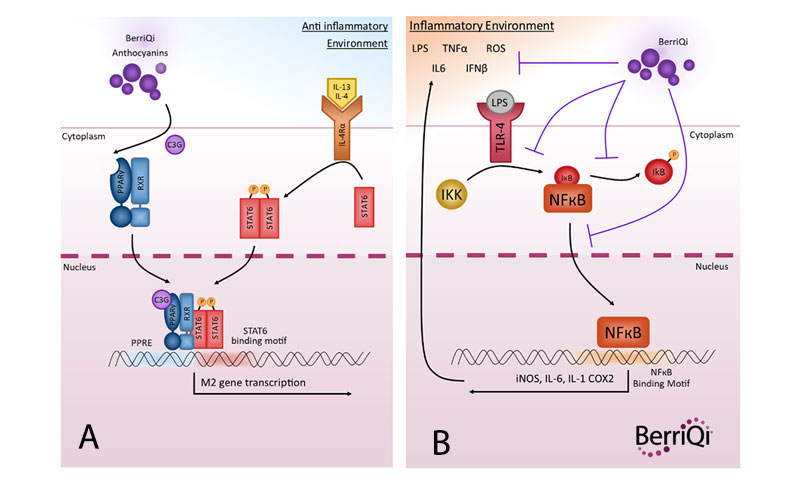
The primary mechanism by which BerriQi actively induces M2 class-switching is likely to be an interaction (ligand binding) between BerriQi anthocyanins and a cell nuclear receptor called the Peroxisome Proliferator-Activated Receptor gamma (PPARγ). This PPARy binding interaction initiates a cascade of signalling pathways inside the cell that culminate in the transcription of M2 genes, and skew the macrophage towards the M2 phenotype (Figure 1A). Another mechanism may be through downregulation of the proinflammatory M1 NFκB pathway, which prevents inflammatory signals from maintaining macrophages in the M1 state (Figure 1B). The M2 macrophages then promote resolution of inflammation and tissue repair and reduction of inappropriate collagen fibrosis.
For more information on macrophage class switching, see:
Ley, K. (2017). M1 means kill; M2 means heal. The Journal of Immunology, 199(7), 2191-2193.