Xeno /ˈzɛnəʊ/ : other; different in origin
Hormesis /’hȯr-ˈmē-səs/ : a favourable biological response to low levels of exposures to toxins or other stressors
Xenohormesis: a favourable biological response to molecules produced in a different organism (ie plants) in response to stressors
We’ve known for a while, either innately or based on evidence, that plants are healthy for humans to eat.
Over the years some discoveries have been made as to what some of those bioactive compounds are, which human systems some plant compounds interact with, and the specific health benefits conferred. We’ve even teased apart many of the molecular targets that these molecules act upon: receptor pathways and enzymes present in humans, leading to vast and varied beneficial downstream effects. But we’re still trying to understand WHY… Why do plants produce these molecules? Why would those molecules benefit humans?
At the tip of the iceberg is co-evolution: We co-evolved with plants and have become dependent on them and their molecules for us to maintain our health. Another theory that builds upon this is the concept of xenohormesis in which molecules that plants produce to protect themselves from stress also confer stress-protection benefits to humans when we consume and absorb them. These molecules may provide information to mammals about the environment or perhaps they cause low levels of stress that activate innate human longevity pathways that help us protect ourselves better.
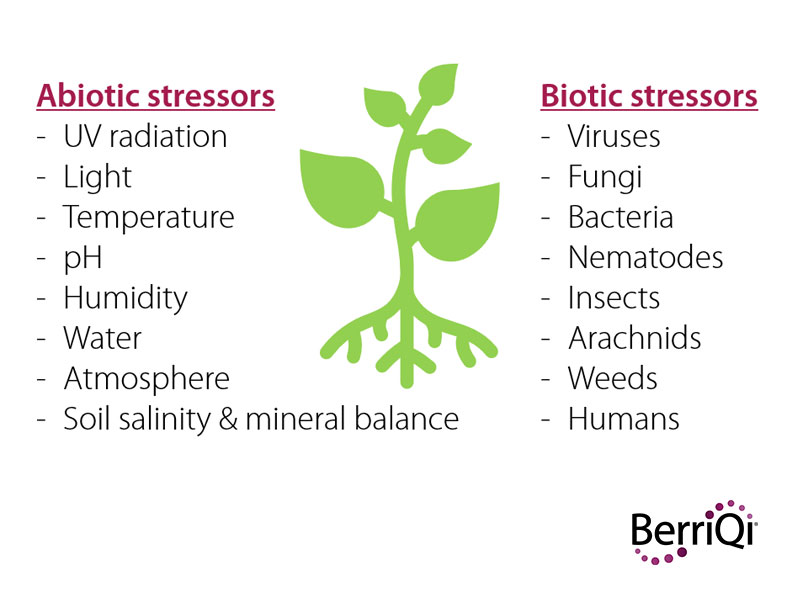
Some abiotic (physical) and biotic (living) stressors that have been found to promote the production of bioactive compounds, also known as plant secondary metabolites, are shown here. For instance, a specific type of anthocyanin called cyanidin-3-O-glucoside, is produced in Boysenberries.
Boysenberries that are grown in New Zealand where UV rays are more intense due to proximity to the ozone hole produce higher concentrations of cyanidin-3-O-glucoside than Boysenberries grown elsewhere on the planet.
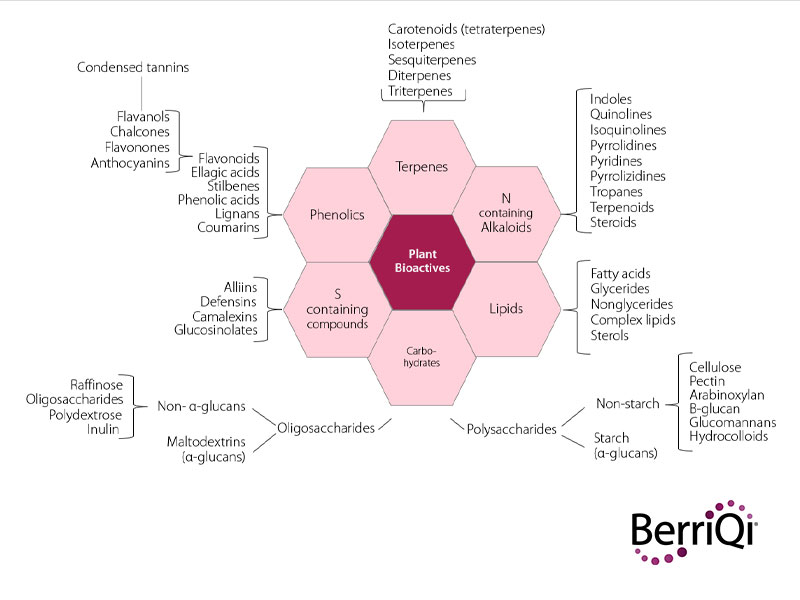
 Considering the immense diversity of plant species on earth, it’s no surprise that there is an even greater diversity of secondary metabolites produced by plants over their lifespan. These fall into 6 broad classes of compounds: phenolics, nitrogen containing alkaloids, sulphur containing compounds, terpenes, lipids, and carbohydrates.
Considering the immense diversity of plant species on earth, it’s no surprise that there is an even greater diversity of secondary metabolites produced by plants over their lifespan. These fall into 6 broad classes of compounds: phenolics, nitrogen containing alkaloids, sulphur containing compounds, terpenes, lipids, and carbohydrates.
Within each of these classes of compounds a vast diversity of sub-classes and unique compounds have been identified. Phenolics have been described as the largest and most diverse class of plant secondary metabolites, but there is much discovery to be done among all classes of bioactives.
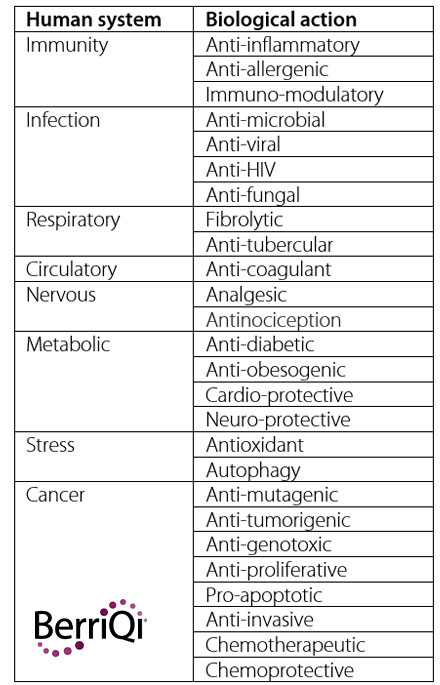
The human systems and biological actions that these bioactive compounds might act upon are also extensive and complex.So far, a pattern has not been established between the class of plant compound and the specific human system affected, as compounds can often interact with and benefit multiple human systems.
For example, the cyaninidin-3-O-glucoside found in New Zealand Boysenberries has been found to have anti-inflammatory, fibrolytic, and antioxidant properties.
And a single species of plant usually expresses several classes of bioactive compounds concurrently. Those same Boysenberries also produce non-starch polysaccharides and condensed tannins which have additional immunomodulatory functions in the human body.
The biological actions of plant secondary metabolites in humans occur through both direct binding pathways and indirect transcriptional modulation, often both, participating in complex feedback loops. Plant secondary metabolites directly bind to human receptors but depending on which cells these receptors are expressed on, the effect may directly change the activity of the cell or could prompt an intra-cellular signalling cascade.
These signalling cascades can either promote or inhibit the translocation of transcription factors, which can either promote or inhibit the expression of proteins, which can totally change the function of a cell type – which may be beneficial or detrimental.
For example, the cyanidin-3-O-glucoside in Boysenberries inhibits the translocation of a pro-inflammatory transcription factor (NFκB), while also promoting the translocation of an anti-inflammatory transcription factor (PPARγ).
In macrophages, tissue-resident immune cells, the anti-inflammatory pathway also promotes the switching from a reactive mode (M1) which is associated with depositing collagen and forming scar tissue, to a healing mode (M2) wherein that scar tissue is broken down and removed.
In the case of lung fibrosis this means the lung elasticity and efficient oxygen and carbon dioxide exchange is restored. When combined with the reduced inflammation from the inhibition of NFκB, healing of fragile lung tissue occurs.
While powerful, the multiple biological targets of plant secondary metabolites, otherwise referred to as molecular promiscuity or pleiotropy, may underpin why the therapeutic index and dose response curve for xenohormetic molecules is fundamentally different than that of pharmaceuticals.
The therapeutic index is considered “the ratio of the highest exposure to the drug that results in no toxicity to the exposure that produces the desired efficacy” (Muller & Milton, 2012) which is identified by using dose response curves, shown below. Whereas pharmaceuticals typically have one molecular target and clearly defined biomarkers, plant secondary metabolites have multiple interrelated targets.
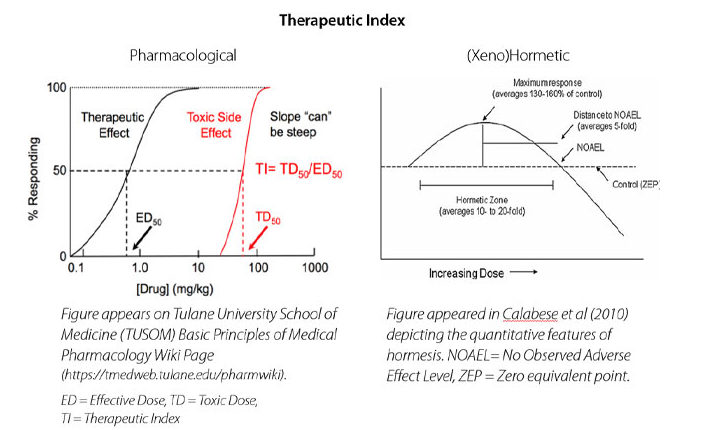
The dose response curve of xenohormetic molecules can make leveraging them for health a counterintuitive exercise, as less is often more… or lower doses may have a more favourable biological effect, as in the Boysenberry cyanidin/macrophage class-switching example given previously. Interestingly, xenohormetic molecules are usually surprisingly safe even at high doses, even if less effective for their intended purpose. At Anagenix our goal is to optimise the amount of bioactive compound in each of our products, based on the clinical evidence for health outcomes.
References:
Aluko, R. (2012). Bioactive carbohydrates. In Functional Foods and Nutraceuticals (pp. 3-22). Springer, New York, NY.
Baur, J. A., & Sinclair, D. A. (2008). What is Xenohormesis?. American journal of pharmacology and toxicology, 3(1), 152–159. https://doi.org/10.3844/ajptsp.2008.152.159
Burow, M., Wittstock, U., & Gershenzon, J. (2008). Sulfur-containing secondary metabolites and their role in plant defense. In Sulfur metabolism in phototrophic organisms (pp. 201-222). Springer, Dordrecht.
Cook, R., & Calabrese, E. J. (2006). The importance of hormesis to public health. Environmental Health Perspectives, 114(11), 1631-1635.
Corson, T. W., & Crews, C. M. (2007). Molecular understanding and modern application of traditional medicines: triumphs and trials. Cell, 130(5), 769-774.
Cory, H., Passarelli, S., Szeto, J., Tamez, M., & Mattei, J. (2018). The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Frontiers in nutrition, 5, 87.
Cummings, J., Stephen, A. Carbohydrate terminology and classification. Eur J Clin Nutr 61, S5–S18 (2007)
da Silva, F. G., Horta, L. P., de Oliveira Faria, R., Stehmann, J. R., & Modolo, L. V. (2014). Stressing conditions as tools to boost the biosynthesis of valuable plant natural products. Recent patents on biotechnology, 8(1), 89–101.
Hayes, M., & Tiwari, B. K. (2015). Bioactive carbohydrates and peptides in foods: An overview of sources, downstream processing steps and associated bioactivities. International journal of molecular sciences, 16(9), 22485-22508.
Hooper, P. L., Hooper, P. L., Tytell, M., & Vígh, L. (2010). Xenohormesis: health benefits from an eon of plant stress response evolution. Cell stress & chaperones, 15(6), 761–770.
Hussein, R. A., & El-Anssary, A. A. (2019). Plants secondary metabolites: the key drivers of the pharmacological actions of medicinal plants. Herbal medicine, 1, 13.
Kurek, J. (2019). Introductory chapter: alkaloids-their importance in nature and for human life. In Alkaloids-Their Importance in Nature and Human Life. IntechOpen.
Mattson M. P. (2008). Hormesis defined. Ageing research reviews, 7(1), 1–7. https://doi.org/10.1016/j.arr.2007.08.007
Oyedepo, T. A., & Kayode, A. A. (2020). Bioactive Carbohydrates, Biological Activities, and Sources. In Functional Foods and Nutraceuticals (pp. 39-74). Springer, Cham.
Selmar, D., & Kleinwächter, M. (2013). Stress enhances the synthesis of secondary plant products: the impact of stress-related over-reduction on the accumulation of natural products. Plant & cell physiology, 54(6), 817–826.
Shaw, O. M., Hurst, R. D., Cooney, J., Sawyer, G. M., Dinnan, H., & Martell, S. (2021). Boysenberry and apple juice concentrate reduced acute lung inflammation and increased M2 macrophage‐associated cytokines in an acute mouse model of allergic airways disease. Food science & nutrition, 9(3), 1491-1503.
Shaw, O. M., Hurst, R. D., & Harper, J. L. (2016). Boysenberry ingestion supports fibrolytic macrophages with the capacity to ameliorate chronic lung remodeling. American Journal of Physiology-Lung Cellular and Molecular Physiology, 311(3), L628-L638.
Vuolo, M. M., Lima, V. S., & Junior, M. R. M. (2019). Phenolic compounds: Structure, classification, and antioxidant power. In Bioactive compounds (pp. 33-50). Woodhead Publishing.
Wink, M. (2015). Modes of action of herbal medicines and plant secondary metabolites. Medicines, 2(3), 251-286.
Zhang, H., & Tsao, R. (2016). Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Current Opinion in Food Science, 8, 33-42.